How Can We Prevent Corrosion In Class 10: Effective Strategies
Prevention Of Corrosion | Science For Grade 10 | Periwinkle
Keywords searched by users: How can we prevent corrosion class 10 5 ways to prevent corrosion, prevention of corrosion class 10 notes, what is corrosion class 10 with examples, what do you mean by corrosion class 10, what do you mean by corrosion class 12, write two ways of prevention of corrosion class 10, what is corrosion of metals how do you prevent corrosion of iron, what do you mean by corrosion how can you prevent it
How Can We Prevent Corrosion?
Preventing corrosion is essential to prolong the lifespan and integrity of various metal structures and components. To achieve effective corrosion prevention, consider the following strategies:
-
Choose the Right Materials: Opt for non-corrosive metals like stainless steel or aluminum when possible. These materials are inherently resistant to corrosion, reducing the risk from the outset.
-
Maintain Clean and Dry Surfaces: Regularly inspect and clean metal surfaces to remove dirt, moisture, and contaminants that can accelerate corrosion. Keeping the surface dry is crucial, as moisture is a key catalyst for the corrosion process.
-
Utilize Drying Agents: Employ drying agents or desiccants in environments prone to humidity. These substances help absorb moisture and maintain a dry atmosphere around metal surfaces.
-
Apply Protective Coatings: Shield metal surfaces with appropriate barrier products like grease, oil, paint, or carbon fiber coatings. These coatings act as a protective layer, creating a barrier between the metal and corrosive elements in the environment.
-
Consider Backfill Materials: In the case of underground piping, consider using a layer of backfill material such as limestone. This backfill material can act as a protective buffer, preventing direct contact between the metal and potentially corrosive soil or groundwater.
By implementing these preventive measures, you can significantly reduce the risk of corrosion and ensure the long-term durability of your metal components and structures.
How Can Corrosion Be Prevented Class 10?
Corrosion prevention, particularly relevant for Class 10 students, is a crucial topic that addresses how certain metals deteriorate when exposed to moisture, acids, and other corrosive agents, leading to the formation of metal oxides on their surfaces. This phenomenon, known as corrosion, can be effectively mitigated through various methods. To protect metal surfaces from corrosion, one can employ techniques such as painting, applying oil or grease, galvanizing, chrome plating, or incorporating alloying elements into the metal composition. These strategies form a protective barrier that shields the metal from harmful environmental factors, ensuring its longevity and functionality. Understanding and implementing these corrosion prevention methods is essential for preserving the integrity and durability of metal objects and structures.
How Can Corrosion Be Prevented Gcse Chemistry?
Preventing corrosion in GCSE chemistry involves employing strategies to shield iron or steel from the harmful effects of oxygen and water. One effective approach is to eliminate exposure to oxygen by safeguarding the metal within an environment filled with inert gases like nitrogen or argon. Additionally, safeguarding against water-induced corrosion can be achieved by storing the metal alongside a desiccant, such as calcium chloride, which absorbs moisture and ensures a dry environment. These corrosion prevention techniques are essential for maintaining the integrity and longevity of iron and steel materials.
Collect 46 How can we prevent corrosion class 10




Categories: Summary 95 How Can We Prevent Corrosion Class 10
See more here: vungtaulocalguide.com

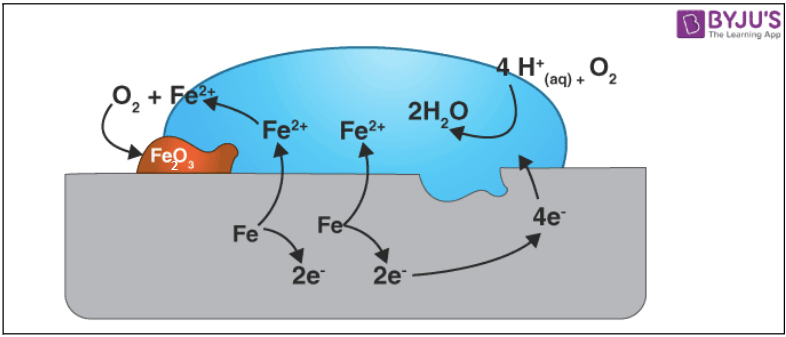
The rusting of iron can be prevented by greasing, painting, galvanizing, anodizing, or oiling the surface. These methods can be classified into the following categories: Galvanization: Galvanized metal is coated with a thin layer of zinc to protect it against corrosion.When some metals are exposed to moisture, acids etc., they tarnish due to the formation of respective metal oxide on their surface. This process is called corrosion. Corrosion can be prevented by painting the surface, oiling, greasing, galvanizing, chrome plating or making alloys.Prevention is defined as the activities taken to prevent a natural calamity or potential hazard from having harmful effects on either people or economic assets.
- Use non-corrosive metals, such as stainless steel or aluminium.
- Make sure the metal surface stays clean and dry.
- Use drying agents.
- Use a coating or barrier product such as grease, oil, paint or carbon fibre coating.
- Lay a layer of backfill, for example limestone, with underground piping.
- oxygen can be excluded by storing the metal in an atmosphere of unreactive nitrogen or argon.
- water can be excluded by storing the metal with a desiccant such as calcium chloride.
Learn more about the topic How can we prevent corrosion class 10.
- Methods for prevention of corrosion – BYJU’S
- What is Corrosion? – Definition and Prevention – TWI Global
- What do you mean by corrosion? How can you prevent it?
- Preventing corrosion – GCSE Chemistry (Single Science) Revision
- Define prevention. – Social Science – Shaalaa.com
- Methods for prevention of corrosion – BYJU’S